The Boy in the Plastic Bubble
On Gene Therapy
👋 Hello, and welcome to Curation for Longevity by Laura Minquini. I am a longevity advocate, entrepreneur, and the founder of MYKIGAI.
In this newsletter, we dive into longevity as a field and practice and look at what can help make it the next consumer health & lifestyle category. 🚀
The following article is the second in our series featuring youth change-makers in longevity! This week, we’re featuring Molly Jin, a 17-year-old interested in gene editing and bioinformatics. In the past, she’s worked as a climate change and gene therapy researcher. She’s aiming to pursue a career in biomedical engineering, focusing on the development of prosthetics. Molly is always up for a challenge and if you have any questions, feel free to reach out to her via email mollyjin2005@gmail.com.
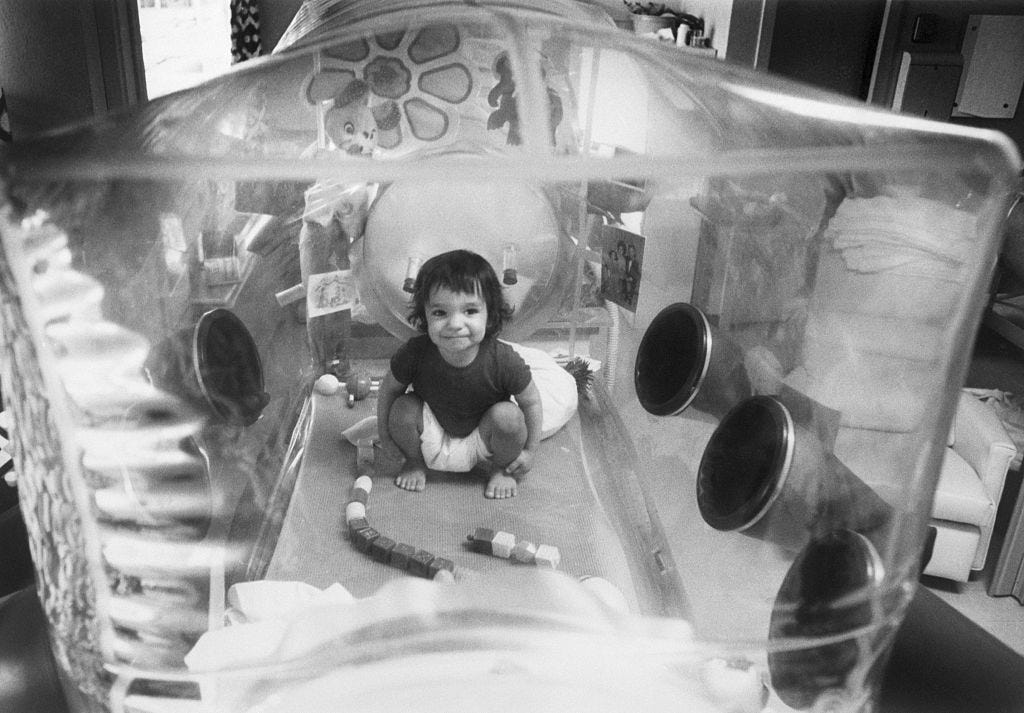
It’s safe to say that the past two years have taught us what it truly means to be separated from other people in society. We have been forced to stay at home for our own safety and we avoided everyone under the assumption that everyone we meet carries COVID-19. Face masks, hand sanitizer, quarantine, lockdowns, social distancing… we are probably sick of all of it.
But what if I came to your house and told you that you have to live like this for the rest of your life, but in a bubble. You would either tell me that I’m a crazy Gen Z and throw a rock at me or accept and live with it. This hypothetical situation would probably never happen to you, but the fact is that there are diseases in the world that force people to self-isolate for the majority of their lives.
Severe combined immunodeficiency (SCID) is a group of rare disorders that are caused by mutations in different genes that are linked to the development and function of immune cells. By the age of 6 months, most infants develop viral infections, candidiasis (a fungal infection caused by a yeast called Candida), Pneumocystis jirovecci pneumonia (an infection caused by the fungus Pneumocystis jirovecii) and diarrhea. As a result, infants with SCID usually die from infections within the first two years of life.
The disease became famously known to the public through David Vetter, who coined the name “Bubble Boy” after he was placed in a germ-free plastic bubble that he lived in for 12 years. After his death due to an unsuccessful bone marrow transplant, public awareness and funding for the research of SCID have dramatically increased.
Diagnosis for SCID is often done through a T-cell receptor excision circle (TREC) test, white blood cell (WBC) count, a history of persistent infections, mitogen and vaccine antigen stimulation assays or even through genetic testing. Clinicians often test patients for common mutations that are characteristic of SCID (eg, IL-2RG, RAG1 and RAG2, JAK3, Artemis [DCLRE1C]) if patients have siblings diagnosed with SCID.
In the late 1990s, testing for SCID was not a procedural test due to the rarity of the disease and because babies with SCID may not show any signs of illness until the development of an infection. Once symptoms arise, it is often too late to take any precautionary measures. Fast forward to today, all states have followed suit to make SCID testing a part of the mandated newborn screening process after Wisconsin passed legislation in 2009.
Positive-tested SCID infants can be given bone-marrow transplants, but the problem with this is that there are racial and ethnic disparities integrated within the organ transplant system, which can make finding a match almost impossible for patients coming from minority communities, as family members are not always necessarily a match. Even if there is a match, a patient’s body can still reject a transplant.
But how does this disease work? How can a disease cause even the slightest bit of human contact to be fatal? What are doctors supposed to do when bone marrow transplants are unavailable or rejected?
The Basics of SCID
SCID consists of defects that target the development of T cells or affect stem cells that feed the lymphoid lineage. The defect is oftentimes combined with a delayed response from antibodies, a Y-shaped protein used by the immune system to identify foreign objects such as pathogenic bacteria and viruses.
Normally, cytokines bind to cytokine receptors on T cells to activate a cascade of intercellular signals for the T cell to differentiate into a mature T cell. In the case of mutation, there is often a defect in cytokine signaling of T cell precursors that are caused by mutations in certain cytokines, cytokine receptors or expression-controlling molecules. This ultimately reduces the number of properly matured T cells in circulation.
SCID is most often caused in two ways: a mutation on the X chromosome or a deficiency in the enzyme adenosine deaminase (ADA).
The Central Dogma
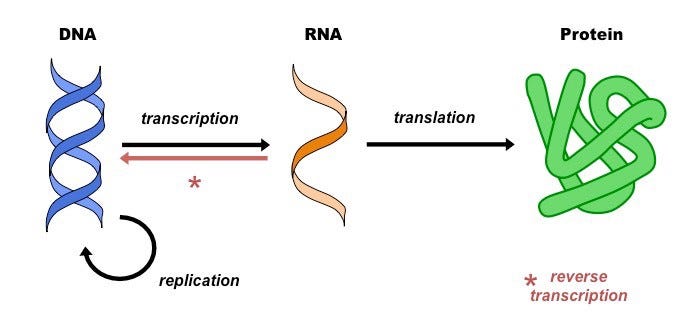
To understand the whole picture, it is important to understand how genes are expressed in the first place. This is done by the Central Dogma, which is the process by which the instructions in DNA are converted into a functional product (kind of like how DNA is any python or java code and the functional product is an output like “Hello World”).
Transcription is the synthesis of RNA using information from the DNA. In this process, the DNA of a particular gene acts as a template for complementary base-pairing. The enzyme, RNA polymerase II, catalyzes the a pre-mRNA molecule, which is then processed to form mature mRNA.
Translation is the process of translating the sequence of a messenger RNA (mRNA) molecule to a sequence of amino acids. Like a book, mRNA is read by a ribosome to produce a chain of amino acids called polypeptides. Three bases in the mRNA constitute a codon and each codon specifies a particular amino acid.
If an individual is born with a defect due to a mutation in its genome, it is almost difficult to change. Most likely, a disease can be treated, but treatment can only go so far. Proteins are the products of translation and in the case of SCID, a lack of ADA being produced due to a mutation can be detrimental to a patient. A mutation on the chromosome would result in the central dogma producing compounds that are not needed for the development of the immune system.
Mutation on the X chromosome
SCID is an autosomal recessive, which means that two copies of an abnormal gene must be present in order for the disease to be expressed. In females, two X chromosomes and in males, there are X and Y chromosomes. Hence, why this disease is more common in males. This form of SCID is most common among SCID-infected patients.
X-linked SCID results from a mutation in the interleukin 2 receptor gamma (IL2RG) gene which produces the common gamma chain subunit, a component of several interleukin (IL) receptors. As seen in the figure to the left, the defect is located on the long arm of the X chromosome at Xq 13. IL2RG is an important gene in the activation of the signalling molecule, JAK3, which helps with the normal development and function of several kinds of immune cells — T and natural killer lymphocytes and nonfunctional B lymphocyte.
Defective IL receptors and IL receptor pathways prevent the development of T-lymphocytes, which is a key player in identifying invading agents as well as activating and regulating other cells of the immune system.
ADA Deficiency
ADA-associated SCID is the second most prevalent form of SCID, occurring in 20% of all SCID patients.
ADA is coded by a gene on chromosome 20 and in most ADA-SCID patients, a mutation on this gene occurs through single base pair substitutions. However, splicing mutations and deletions have been observed. This means that during transcription and translation, ADA is not present in high concentrations.
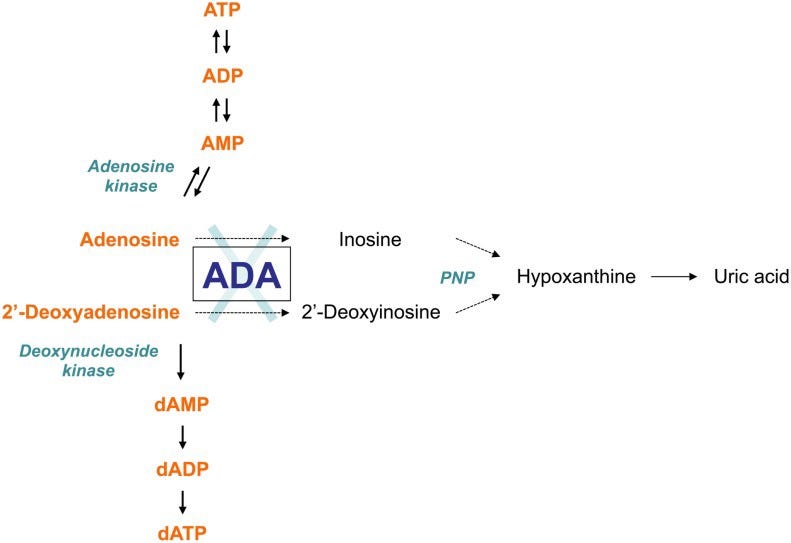
ADA is a key enzyme in the purine salvage pathway, which is a sequence of enzyme-catalyzed reactions in the purine metabolism that uses preformed purine bases or nucleosides to form new nucleotides. ADA catalyzes the deamination (the removal of an amino group from a molecule) of adenosine, 2'-deoxyadenosine and several naturally occurring methylated adenosine compounds. Through deamination of adenosine and 2'-deoxyadenosine, the reaction yields inosine and deoxyinosine, respectively. Further conversion of the deaminated nucleosides results in hypoxanthine, which can be either transformed irreversibly into uric acid or salvaged into mononucleoside.
ADA is present in all cell types of the body, but the highest amounts are found in lymphoid tissues, particularly the thymus, the brain, and gastrointestinal tract. This means that an accumulation of its substrates has a detrimental effect on the state of these tissues.
A deficiency in ADA would result in the toxic accumulation of its substrates (adenosine and 2'-deoxyadenosine). Enzymes have the ability to catalyze a reaction at a much higher speed, so a lack of products would result in the premature death of the lymphoid lineage. Immature lymphoid cells of the immune system are particularly sensitive to the toxic effects of these unused substrates, so they then fail to reach maturity. Thus, the immune system of the afflicted individual is severely compromised.
2'-deoxyadenosine is derived from the breakdown of the DNA molecule and thus, the concentration of 2'-deoxyadenosine is expected to be the highest during cell death. 2'-deoxyadenosine functions as cytotoxic metabolite, which leads to lymphotoxicity in most ADA-SCID patients. Due to the uptake of increasing concentrations of 2'-deoxyadenosine, there is an accumulation of amounts of dATP in erythrocytes and lymphocytes. High amounts of dATP can result in it being a potent, general inhibitor of nucleotide reduction.
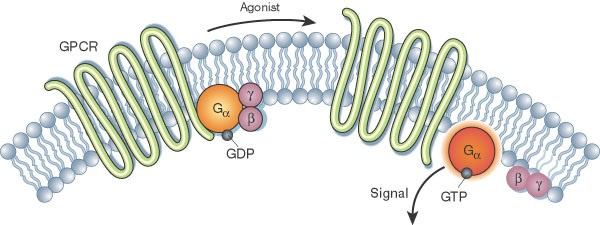
In ATP and RNA, adenosine is a component of the adenine nucleotides. High concentrations of adenosine contribute to apoptosis (programmed cell death) and act as an inhibitor in the differentiation of thymocytes, causing severe T lymphopenia. Adenosine functions as an extracellular signal transducer on cell surface G protein-coupled receptors in which adenosine regulates many physiological processes such as sleep and hearing protection. Adenosine is also involved in neurotransmission, the control of heart rate and blood pressure, and renal function. It is then evident that accumulation in this substrate has many spillover effects to other physiological processes that are essential to our survival.
HIV Gene Therapy
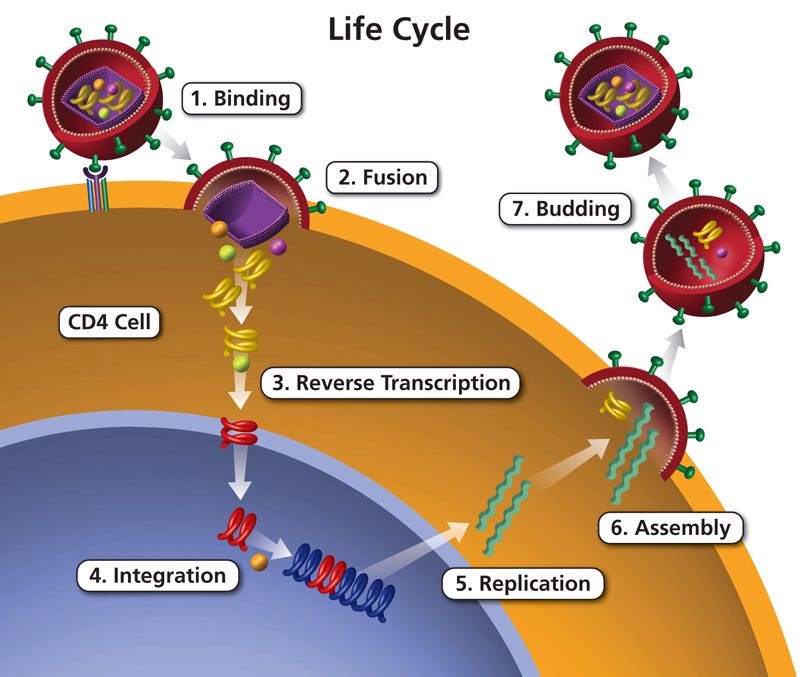
Human immunodeficiency virus (HIV) is a retrovirus, which means that instead of injecting DNA into its host cell, the virus injects RNA instead. Ultimately, HIV has the potential to change a host cell’s entire genome. HIV is known by most people as the virus that attacks the immune system, which most oftentimes leads to the development of AIDS.
HIV infects T-helper cells (a.k.a CD4 cells) in the immune system. Like many other viruses, HIV cannot produce on its own and requires a host cell. The virus attaches itself to a T-helper cell and fuses with it. Because HIV is a retrovirus, reverse transcriptase, which is a DNA polymerase enzyme that transcribes single-stranded RNA into DNA, is used in order for the virus’s DNA to be integrated into the host cell’s genome. It then makes copies of itself and releases the new copies of HIV into the bloodstream to infect other T-helper cells.
So it becomes slightly ironic when the virus is used to cure an already weakened immune system. However, HIV’s metabolic process is beautiful and intricate, which can be exploited to provide a cure for SCID patients. Of course, HIV is deactivated so that all its harmful properties are unable to infect a patient. In this study, this gene therapy technique is being tested on mice.
The process in which a retrovirus can alter a cell’s genome is what can be exploited. Scientists were able to develop a high-titer amphitropic retroviral vector for the transfer of IL2RG (gamma c). The viral vector was used to transfer a copy of the IL2RG complementary DNA (cDNA) to murine 3T3 fibroblasts, which are several cell lines of mouse embryonic fibroblasts, and CD34-enriched hematopoietic progenitor cells obtained from the bone marrow and umbilical cord blood of donors. These cells are then to be transplanted murine bone marrow progenitors.
Murine 3T3 cells transduced by the retroviral vector were analyzed by techniques such as the Southern blot hybridization and Western blot transfer.
Southern Blot Hybridization
Southern blotting is a laboratory technique used to detect a specific DNA sequence in a blood or tissue sample. How it works is that firstly, a restriction enzyme is used to cut a sample of DNA into fragments that are separated using a technique called gel electrophoresis, which separates mixtures of DNA, RNA, or proteins according to molecular size.
The DNA fragments are then transferred to the surface of a membrane. The membrane is exposed to a DNA probe labelled with a radioactive tag. If the probe binds to the membrane, then the desired sequence is present in the sample, indicating that a DNA sequence has been successfully integrated.
Western Blot Transfer
The Western Blot Transfer technique involves the separation and identification of certain proteins. A mixture of proteins is separated based on molecular weight, which allows it to be separated by type, through gel electrophoresis. These results are then transferred to a membrane producing a band for each protein in which afterwards, the membrane is then incubated with labels antibodies specific to the specified proteins.
So… did it work?
Southern blot analysis showed the integration of unrearranged proviral DNA. Western blot analysis demonstrated the expression of gamma c protein through the detection of this protein’s molecular weight. CD34-enriched cells were infected with the viral vectors containing IL2RG and they were then grown in a methylcellulose media. After a period of 2 weeks, individual colonies were analyzed by a polymerase chain reaction assay, which confirmed the proviral marking.
Another application that was used in this study was the use of the vector to transfer a copy of the gamma cDNA to murine bone marrow cells in a transplantation model. The bone marrow was then transplanted into syngeneic Balb/c mice.
Through a recent clinical trial, nearly 50 X-linked SCID infants developed a well-functioning immune system through this technique. A follow-up on all of these infants is currently being conducted, but most have been able to live a normal lifestyle.
Using Viruses In Gene Therapy
In the fascinating field of gene therapy, many types of viruses such as retrovirus, adenovirus, adeno-associated virus (AAV), and herpes simplex virus, have been modified. All of these viruses involve the integration and change of a cell’s genome which can cure many of the diseases we consider to be fatal today, as viruses are able to conduct tasks at a molecular level.
It is fascinating and exciting how something that is considered harmful can be transformed into something that is used to cure diseases we deemed impossible to cure. This opens the door to other forms of viruses and even bacteria as well. The irony behind this story shows the magnitude of the advancements in science and shows that science will continue to develop exponentially.
Select References: